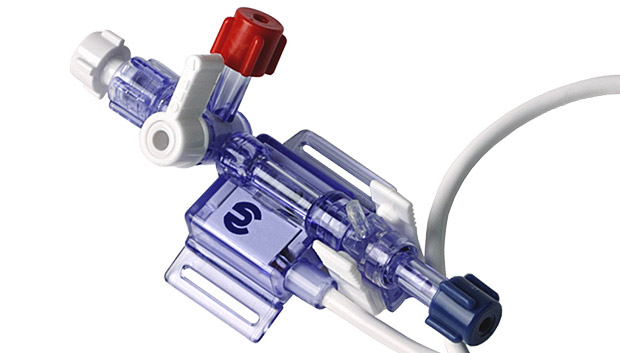
In critical care situations, when quick changes in blood pressure are expected or when a critically ill patient requires long-term blood pressure measurement and blood sampling, use of the Deltran® invasive blood pressure monitoring system provides the accuracy of measurement at the arterial source and the convenience of continual pressure measurement and access to the blood source. The procedure involves placing a cannula needle into an artery. This intra-arterial cannula is connected to a fluid-filled system of tubing where a solid column of fluid connects arterial blood to the Deltran® disposable pressure transducer. The transducer is connected to a pressure monitor that displays the blood pressure data graphically on screen and chart paper.
PRODUCT DESCRIPTION
The Deltran® is a Disposable Pressure Monitoring System with integral flush device and stopcock.
FEATURES
INDICATIONS
The Deltran® Disposable Pressure Monitoring System is intended for use in physiological pressure measurements which require continuous flow to maintain catheter patency.
For more information, please contact us by EMAIL or call 1-800-533-4984 | 1-801-566-1200.
© Copyright 2024 Utah Medical Products, Inc. All rights reserved.