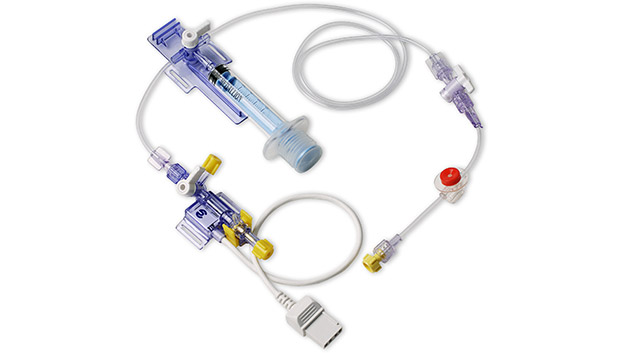
During invasive blood pressure monitoring, clinicians often prefer to draw arterial blood samples through the transducer's high pressure tubing. Clinicians prefer this method because it takes advantage of the catheter that is already in place. However, taking samples directly from stopcocks on the high pressure tubing line opens the system to possible infection from the outside and exposes the clinician to blood. The Deltran® Plus needleless arterial blood collection system incorporates an entirely closed system using needleless technology and the neonatal version of the system is designed specifically to conserve blood and protect the neonate from infection.
As a closed needleless system, Deltran® Plus gives the clinician the confidence of absolute integrity throughout the monitoring process. The integrated reservoir syringe and needleless blood sampling port provide significant benefits.
PRODUCT DESCRIPTION
The Neonatal/Pediatric Deltran® Plus System is a needleless, closed, in-line blood sampling and pressure monitoring system intended for use in neonatal and pediatric applications and incorporates a 30cc/hour flush device.
FEATURES
INDICATIONS
The Deltran® Disposable Pressure Monitoring System is intended for use in physiological pressure measurements which require continuous flow to maintain catheter patency. Deltran® Plus is used to draw arterial blood samples through the Deltran® transducer's high pressure tubing.
For more information, please contact us by EMAIL or call 1-800-533-4984 | 1-801-566-1200.
© Copyright 2024 Utah Medical Products, Inc. All rights reserved.